Gas Laws Worksheet
Select "data" from the task bar in the top left, then select "new. What change in volume results if 60.0 ml of gas is cooled from 33.0 °c to 5.00 °c?

Worksheet On Ideal Gas Equation / Combined Gas Law
Fun with dalton's law o' partial pressures!

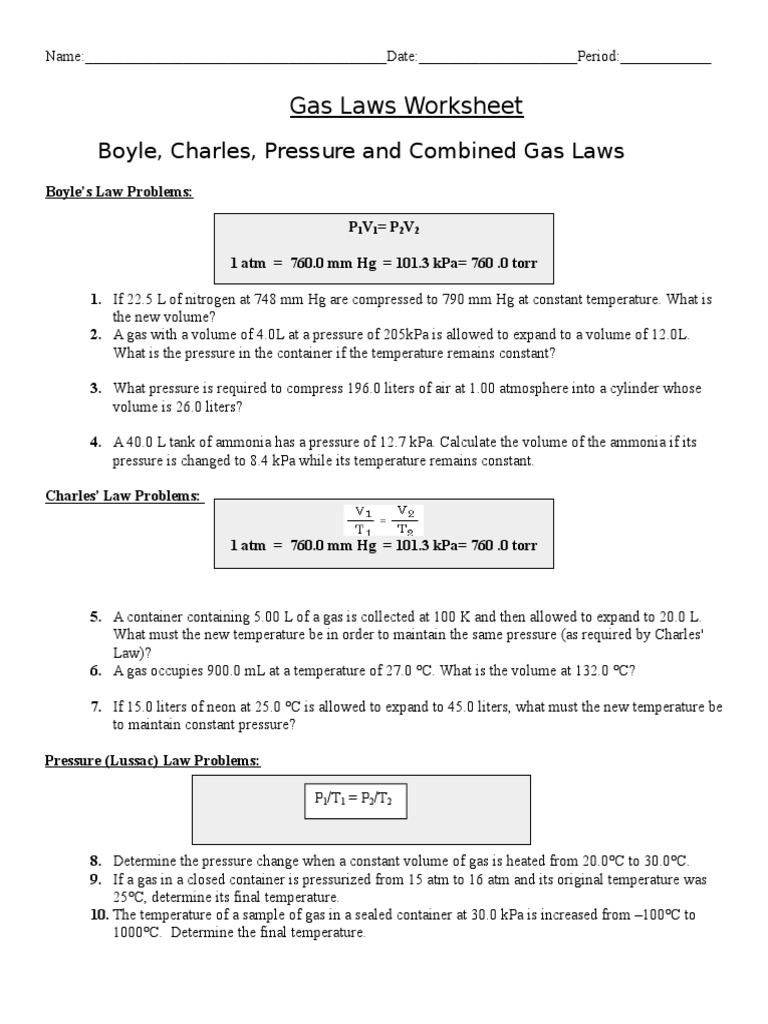
Gas laws worksheet. The volume of a gas varies linearly with the number of moles: If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. Or try this gas law wordsearch puzzle ( doc) with answers ( doc).
Student worksheet for chemical gas laws attempt to work the following practice problems after working through the sample problems in the videos. N = pv = (2.8 atm)(98 l) = 11 moles of gas rt (0.0821 l.atm/mol.k)(292 k) 2) if 5.0 moles of o 2 and 3.0 moles of n 2 are placed in a 30.0 l tank at a temperature of 25 0 The simulation (15 minutes) we are going to study 2 of the famous gas laws:
Gases add to my workbooks (5) add to google classroom add to microsoft teams share through whatsapp: By amanda on february 20, 2022. Sketch on the graph below how.
What is the new volume? A good worksheet for helping the students to figure out when to use each law. Gases worksheet 1 by tassiebadenhorst:
To correctly analyze and apply the data obtained in part b, all temperatures must be converted to kelvin (k) from degrees celsius (°c). More gases laws interactive worksheets. Σ ������ k i������������ ������.
Determine the volume of this sample at 760 mm hg and 37°c. Gas laws worksheet 1 bo le s charles ga lussac s and combined gas law solve all problems you must show your work including units. Boyle's law, which looks at the relationship between pressure and volume, and charles's law, which looks at the relationship between volume and temperature.
Answers are given on the last page(s). Word problems based on the ideal gas law. Gases worksheet 3 by tassiebadenhorst:
A sample of gas occupies a volume of 450.0 ml at 740 mm hg and 16°c. Ideal gas law worksheet #1: Relevant equations gas laws moles and rates boyle's law:
If the initial pressure of the gas is 145 atm and if the temperature February 20, 2022 on ideal gas law practice problems worksheet answers. Dalton + gases + practice = fun!
A sample of gas is transferred from a 75 ml vessel to a 500.0 ml vessel. Do this gas laws crossword puzzle ( doc) or try this "gases" ( pdf) crossword with answers. What is the volume of hydrogen at a pressure of 1.06 atm if 200 cm3 of hydrogen is collected at a pressure of 1.00 atm?
V = k a × n k a is avogadro's gas constant • these are unified in the ideal gas law: Some of the worksheets for this concept are mixed gas laws work, gaslawssupplementalwork, unit 7 the gas laws, unit conversions for the gas laws, ap chemistry review work unit 5 the gas laws, the ideal gas law chem19013 work 8 the ideal gas, work 7, name date gas laws. The volume changes from 45 l to 40 l.
Simulation worksheet 2 screen 3: Chemistry gas law's worksheet 10. 1 atm = 760.0 mm hg = 101.3 kpa.
Sketch on the graph below how the volume of a gas changes as the pressure is increased. Gas laws worksheet atm = 760.0 mm hg = 101.3 kpa= 760.0 torr boyle's law problems: Ideal gas law worksheet #2:
Gases worksheet 4 by tassiebadenhorst: Gas law worksheet #2 (dalton's law and ideal gas law) dalton's law: The pressure of a gas changes from 120 kpa to 50 kpa.
About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Gas laws worksheet npsd k12 nj us laws worksheet atm 760 0 mm hg 101 3 kpa 760 0 torr boyle acirc euro trade s law problems 1 if 22 5 l of nitrogen at 748 mm hg are pdf document. A gas occupies 900.0 ml at a temperature of 27.0 °c.
P t = p 1 + p 2 + p 3 +. This worksheet ( doc) is a review of all the gas laws. Have students try this "gas laws magic square" ( doc).
Gas laws ideal gas law homework ideal gas law chemistry classroom science chemistry. Pv = nrt p = pressure v = volume n= moles of gas, r = universal gas constant t = temperature. If 22 5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature.
Molar mass from molar volume avogadro s law a2. The value of r varies with the units chosen: If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature.
To convert to units of k, create a new data column: Determine the total pressure of a gas mixture that contains oxygen at a pressure of 150.mmhg, nitrogen at 350.mmhg pressure, and helium at a pressure of 200.mmhg. Use excel or logger pro 3.14 to open the file you saved from part b.
If 400 cm3 of oxygen is collected at a pressure of 980 mmhg, what volume will the gas occupy if the pressure were changed to 940 mmhg? A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. A gas mixture containing oxygen, nitrogen, and carbon dioxide has a pressure of 250.mmhg.
What is the volume at 132.0 °c? P 1 v 1 = p 2 v 2 molar mass: A question on a university of washington midterm was, "is hell exothermic?"
The ideal and combined gas laws: If a gas at occupies 2.60 liters at a pressure of 1.00 atm, what will be its volume at a pressure of 3.50 atm? Pv = nrt r is the universal gas constant critical thinking questions 1.
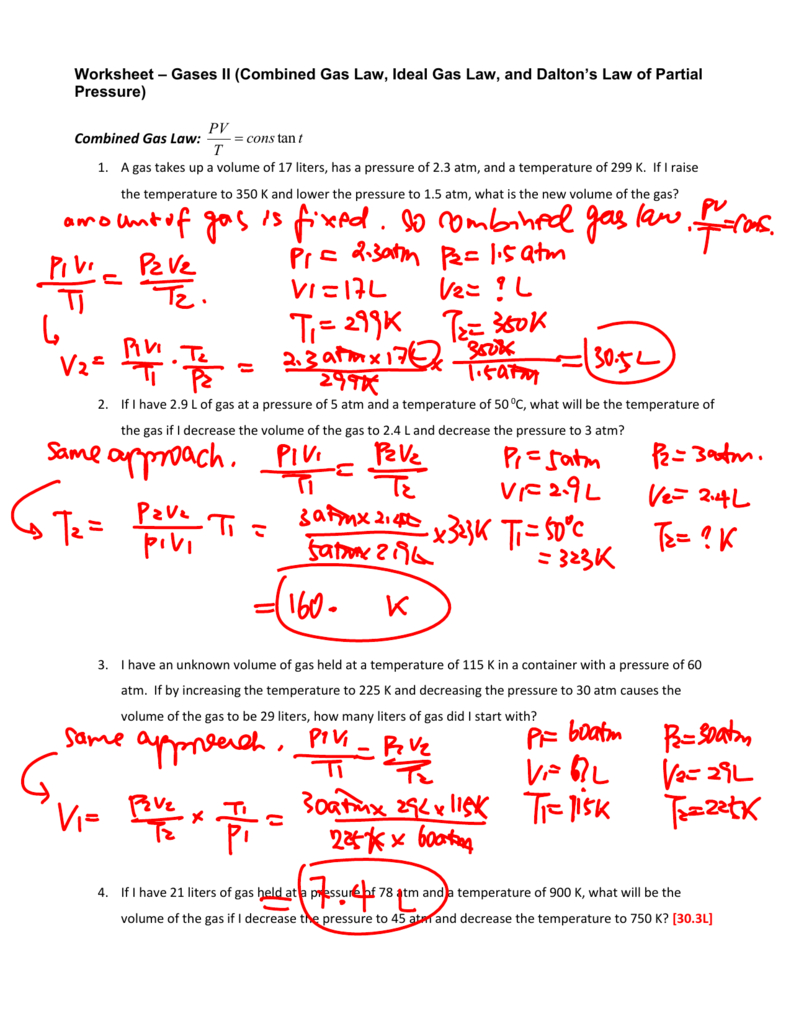
Combined Gas Law Worksheet Answer Key —
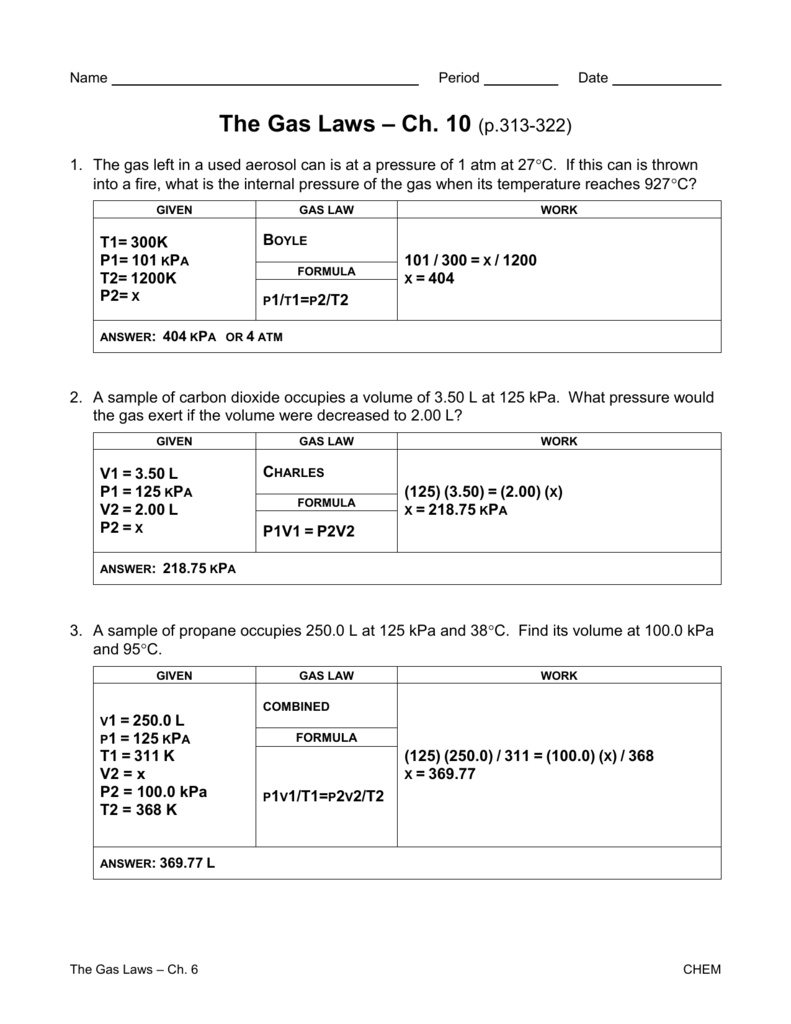
Gas Laws Worksheet 1 Answer Key —
32 Gas Laws Worksheet With Answers Worksheet Project List

Combined Gas Law Problems Worksheet

Gas Laws And Scuba Diving Worksheet Answers —
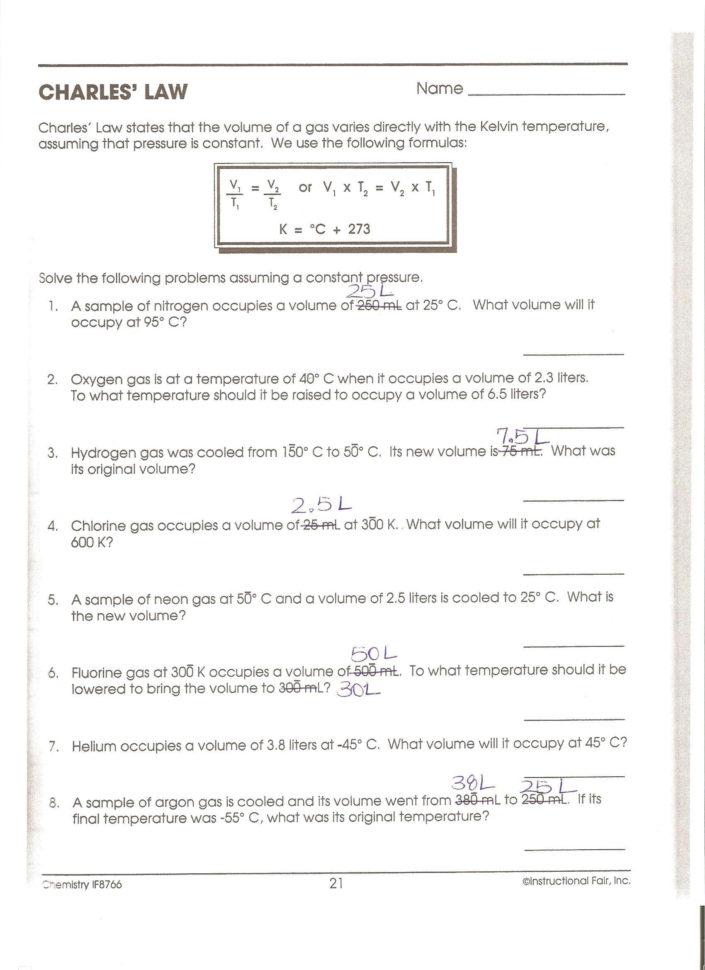
Worksheet Mixed Gas Laws Worksheet The Gas Laws Worksheet

Combined Gas Law Worksheet Answer Key —

Ideal Gas Law Worksheet Answer Key
Gas Laws Worksheet Answer Key Gases Litre

Chemistry Gas Laws Worksheet Answers

Combined Gas Law Worksheet Answer Key
Gas Laws Worksheet 2 Boyles Charles and Combined PDF

Ideal Gas Law Worksheet Answers Chemistry If8766 worksheet

Combined Gas Law Worksheet Answer Key —
13 Best Images of Pressure Problems Worksheet Answer Key